Mycoplasma and House Dust Mites; the tiny troublesome duo
Mycoplasma’s are the nightmare of every laboratory personnel where sometimes it seems they can’t wake up from. They are responsible for cell culture contamination which can lead to negative influence on the research results. Don’t be startled if you found out about Mycoplasma’s for the first time at your laboratory, because they are responsible for up to 60% of the contamination of cell culture. So, after reading the title, you may think; what do mycoplasmas have to do with House Dust Mites? This article will explain more about these parasites, how they are linked to HDM’s and of course; how can you wake up form this nightmare?
Background
With Citeq being a producer of House dust mite (HDM) materials and source materials, our products are also used in cell based assays. A possible contaminant of the HDM products was noticed at the cell culture laboratory at Citeq. During the cell culture analyses it was observed that cell growth was inhibited, but the cause was unknown. A well-known genus of bacteria, the Mollicute class, are known to be one of the most common cell culture contaminants. As a result, the cell culture was tested for the presence of Mycoplasma using the famous PCR technique. Results showed that the cell culture was positive for Mycoplasmas, which means that the cells were indeed infected. In this way the suspicion arose that Mycoplasmas could be present in the mites and mite (source)materials.
After these findings, all our products were tested for mycoplasmas by PCR and cultivation on agar. The important part of the testing was to find out if the mycoplasmas are viable, since this form can cause infections. The PCR method can’t distinguish viable and non-viable mycoplasma but cultivation on agar makes sure to also detect the viable mycoplasma. Results showed that mycoplasma was detected by PCR but also by agar, which means that the HDM’s contain active mycoplasma bacteria. Being pathogenic, these bacteria could cause infections in humans during diagnostic and research applications with these products. This topic has not been extensively researched before and Citeq is the first to discover these findings.
What are mycoplasmas?
Mycoplasmas belong to the Mollicute class and are the smallest known bacteria that lack a cell wall making them resistant to a lot of common antibiotics and many are parasitic in nature, attaching to- and eventually invading other cells. Their hosts are all living species, for instance humans, animals (including Arthropods) and plants. This means a contamination of a model with mycoplasma is difficult to detect. With their size of 0.1 µm, it is easy for them to infect mammal, plant but also arthropod hosts (Kumlert et al., 2018; Maggi et al., 2013), which makes them a potential contaminant of our products since HDMs belong to the arthropod class (see Figure 1). The next paragraph will explain the hosts that are applicable to HDM’s.
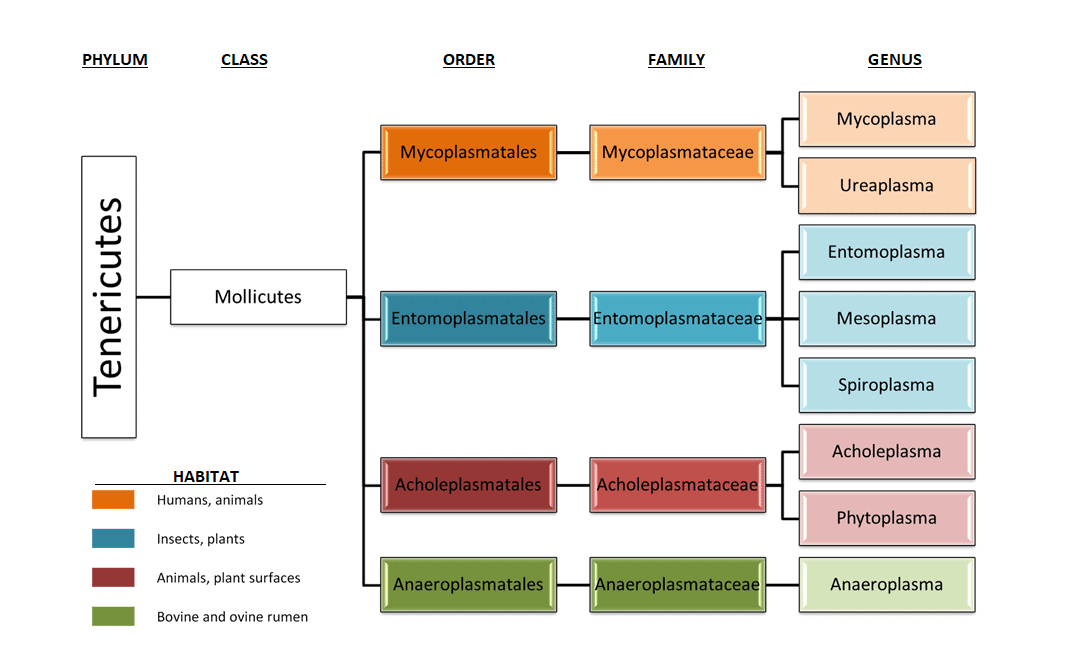
paragraph will explain the hosts that are applicable to HDM’s. Figure 1; Taxonomy overview of the Mollicute class and their hosts.
Mycoplasma in mouse models and in humans
Mouse models
Multiple research groups from universities all over the world are using different murine models for asthma research. Studies have shown that 5 different mycoplasma species are identified in laboratory mice. These 5 species include M. arthritidis, M. collis, M. muris, M. neurolyticum and M. pulmonis with mice and rats as primary hosts. (Masoumalinejad et al., 2018). Infection with these Mycoplasmas has effect on immune responses and may predispose to other infections and can also alter the physiological response of mice in experiments. Animals with this infection may be clinically ill, which makes them unfit for use. Transmission of these strains occurs through direct contact and aerosol (Homberger & Thomann, 1994, p. 118).
Masoumalinejad, Z., Zinatizadeh, M. R., & Tahmasebiabdar, N. (2018). A Review of Mycoplasma in Laboratory Mice. Modern Medical Laboratory Journal, 02(01), 15–19. https://doi.org/10.30699/mmlj17.2.1.15Homberger, F. R., & Thomann, P. E. (1994). Transmission of murine viruses and mycoplasma in laboratory mouse colonies with respect to housing conditions. Laboratory Animals, 28(2), 113–120. https://doi.org/10.1258/002367794780745263
Humans
Mycoplasmas are prevalent on mucosal surfaces of the urogenital and respiratory tracts of humans and animals. Despite being host-dependent for nutrients, at least 4 Mycoplasma species (see Table 1: (Razin, 2002)) are considered human Mycoplasmas and 20 have been identified as cell culture contaminants (Nikfarjam & Farzaneh, 2012; WHO, 2019). The 4 pathogenic species are described in in the following paragraph:
- M. pneumoniae; is a well-recognized Mycoplasma specie that often gives rise to pneumonia in children and young adults (Sutherland, Brandorff, & Martin, 2004), which can lead to a mild respiratory tract infection or a severe pneumonia. They can only survive by adhesion to the epithelium of the respiratory tract by adhesion proteins (Gillespie et al., 2015). The expectation is that this M. pneumoniae is present in the HDMs, because they also play a role in respiratory infections.
- M. hominis; is related to genital infections in men, gynaecological infections and pregnancy related disorders (Blazek, Schmitt, Krafft, & Hadding, 1990; Uphoff & Drexler, 2002)
- M. genitalium; is a sexually transmitted Mycoplasma specie of which the clinical relevance has only recently been recognized. It can lead to urethritis and infertility (Herrmann, 2002).
- M. fermentas; can be found in the genital area and is linked to rheumatoid arthritis. Some strains can lead a to respiratory infection that harms other organs (Yáñez et al., 2013).
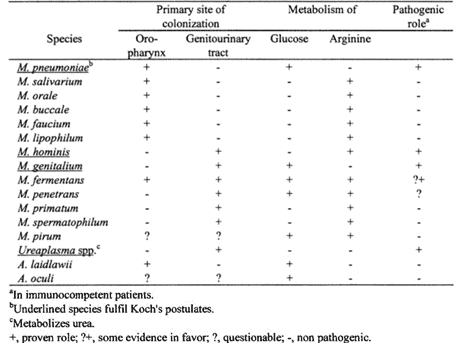
Note. Reprinted from “Molecular Biology and Pathogenicity of Mycoplasmas”, by Razin, S., 2002, p. 47, New York, NY: Free Press. Copyright 2002 by Kluwer Academic Publishers.
What now?
It is important to realize that mycoplasmas are present in not only in cell cultures but also in the products made from the HDM’s; D.pteronyssinus and D.farinae you are using because it can affect your research. So the first question that comes up in your mind now is probably: what is the cause of this contamination? Well, mycoplasmas can enter their host very easily by aerosols with humans being the largest source of contamination. This contamination is most of the time a consequence of poor lab technique and human actions such as sneezing, coughing but also talking and touching materials.
Based on these findings, the central question is: ‘How can we detect Mycoplasma in HDMs and HDM products?’. By finding out which Mollicute species are pathogenic, which detection methods are available and how this can be applied to our products, we made a testing protocol for the products to ensure their quality. The next paragraph will explain the method of testing for Mollicutes.
Testing for mycoplasmas
Most of our new house dust mite extracts are analysed on the presence of mycoplasma. We have used the PCR method for the detection of Mollicute species, since it is the most quick and used method. Another detection method that has been tested is by cultivation on agar and is called the ‘golden standard’. The agar medium (Mycoplasma-G) is very complex and there is no medium available where all known species can grow on (EMA-quality, 2013; Molla Kazemiha et al., 2009). It is possible that de PCR only detects non-viable mycoplasma so this is the reason why our products have also been cultivated on agar. Cultivation on agar showed that the whole culture products contained living mycoplasma, but the extracts were free of mycoplasmas. It is therefore important to take this in consideration by using the whole culture for your experiments.
The results of this test can also be found on our certificates of analysis. Our products are tested for the following species:
| Mollicute species being tested by PCR | |
| M.arginine | M.salivarium |
| M.orale | U.urealyticum |
| M.hyorhinis | M.synoviae |
| M.fermentans | M.bovis |
| M.genitalium | M.arthritidis *a |
| A.laidlawii | M.collis*a |
| M.hominis | M.muris*a |
| M.pirum | M.neurolyticum*a |
| M.pneumoniae | M.pulmonis*a |
*a Mollicute specie that is a murine host
Conclusion
Based on what has been discussed in this article it is better too be safe then sorry.
Our recommendation is to prevent contamination by the following;
- Wear clean lab coat and gloves
- Screen your source material by testing for mycoplasmas
- Make sure to use proper sterile teqnchique and to keep your work space clean
- Screen for mycoplasma routinely at the start of. Any experiment, and prior to publication
- Instruct others to prevent mycoplasma contamintation
The PCR method can’t distinguish viable and non-viable mycoplasma but cultivation on agar makes sure to also detect the viable mycoplasma. If the product that is going to be used has to be free of mycoplasma, our advise is to use the two mentioned detection methods; PCR and cultivation on agar.
If you wish for a more detailed explanation on the prescense of mycoplasmas in HDM’s, check out our poster; ‘Detection of Mollicute species (Mycoplasma) in House Dust Mites’ presented on the EAACI and ISMA in 2019
