Scientific publications
Scientific publications
Products of Citeq Biologics are used by scientists all over the world. Besides active collaborations with multiple research groups our products are cited in articles which will be listed here but can also be found on Google Scholar.
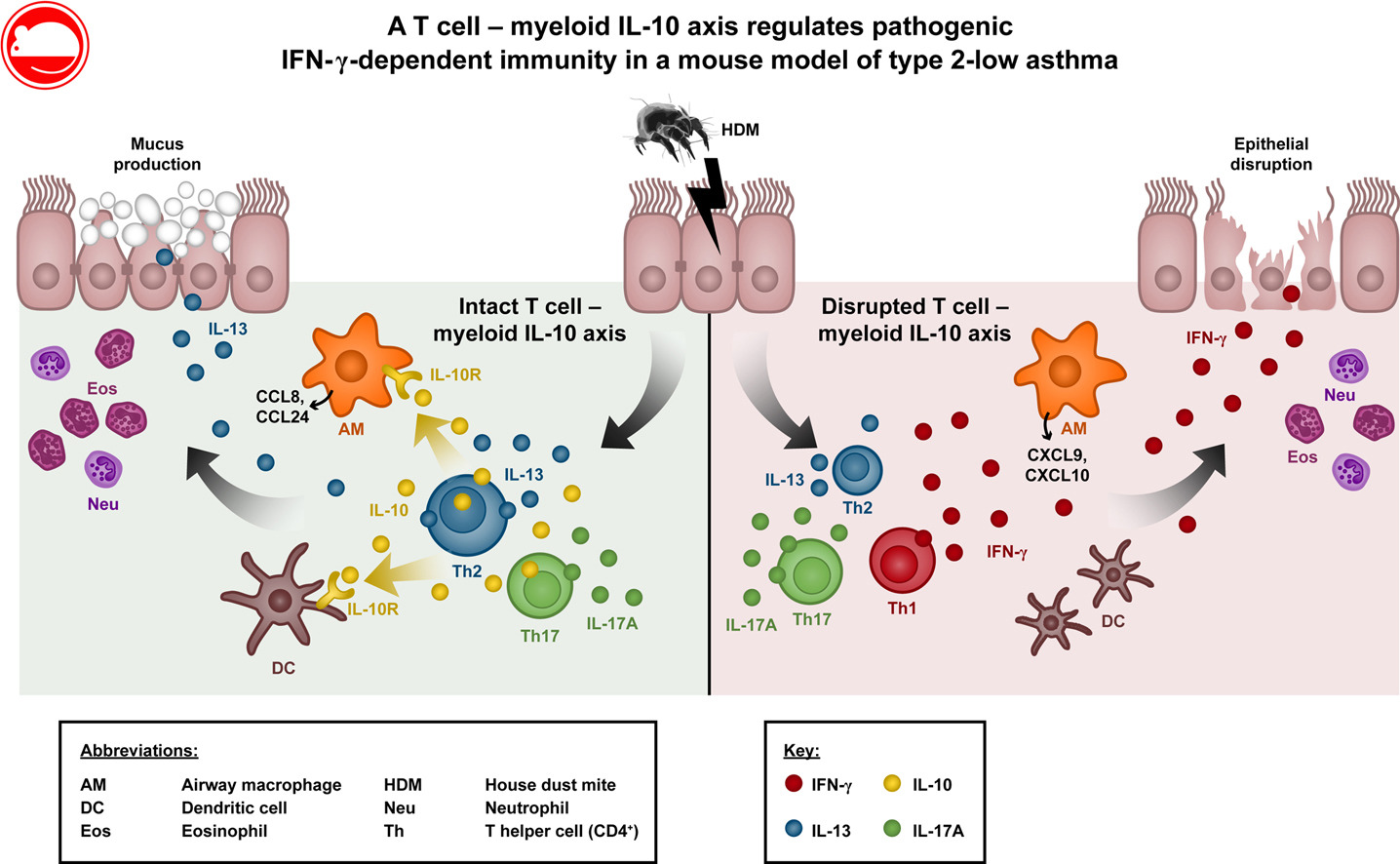
A T cell – myeloid IL-10 axis regulates pathogenic IFN-y dependent immunity in a mouse model of type 2-low asthma

Published August 22, 2019 Research group of Clare M. Lloyd Institution: Imperial College London
Background Although originally defined as a type 2 (T2) immune-mediated condition, non-T2 cytokines, such as IFN-γ and IL-17A, have been implicated in asthma pathogenesis, particularly in patients with severe disease. IL-10 regulates TH cell phenotypes and can dampen T2 immunity to allergens, but its functions in controlling non-T2 cytokine responses in asthmatic patients are unclear.
Objective We sought to determine how IL-10 regulates the balance of TH cell responses to inhaled allergen.
Methods Allergic airway disease was induced in wild-type, IL-10 reporter, and conditional IL-10 or IL-10 receptor α (IL-10Rα) knockout mice by means of repeated intranasal administration of house dust mite (HDM). IL-10 and IFN-γ signaling were disrupted by using blocking antibodies.
Results Repeated HDM inhalation induced a mixed IL-13/IL-17A response and accumulation of IL-10–producing forkhead box P3–negative effector CD4+ T cells in the lungs. Ablation of T cell–derived IL-10 increased the IFN-γ and IL-17A response to HDM, reducing IL-13 levels and airway eosinophilia without affecting IgE levels or airway hyperresponsiveness. The increased IFN-γ response could be recapitulated by IL-10Rα deletion in CD11c+ myeloid cells or local IL-10Rα blockade. Disruption of the T cell–myeloid IL-10 axis resulted in increased pulmonary monocyte–derived dendritic cell numbers and increased IFN-γ–dependent expression of CXCR3 ligands by airway macrophages, which is suggestive of a feedforward loop of TH1 cell recruitment. Augmented IFN-γ responses in the HDM allergic airway disease model were accompanied by increased disruption of airway epithelium, which was reversed by therapeutic blockade of IFN-γ.
Conclusions IL-10 from effector T cells signals to CD11c+ myeloid cells to suppress an atypical and pathogenic IFN-γ response to inhaled HDM.
Key words Severe asthma, type 2–low asthma, IL-10, immune regulation, T cell, macrophage, dendritic cell, IFN-γ
Read the full paper here.
Published July 19, 2019 Research group of Arno C. Gutleb Institution: Luxembourg Institute of Science and Technology
The aim of the study was to develop an in vitro model that mimics the alveolar-capillary barrier and that allows assessment of the respiratory sensitizing potential of substances. The 3D in vitro model cultured at the air liquid interface consists of alveolar type II epithelial cells (A549), endothelial cells (EA.hy926), macrophage-like cells (PMA-differentiated THP-1), and dendritic-like cells (non-differentiated THP-1). This alveolar model was exposed apically to nebulized chemical respiratory sensitizers (phthalic anhydride (PA) and trimellitic anhydride (TMA)) or to irritants (methyl salicylate (MeSa) and acrolein (Acr)) at concentrations inducing 25% cytotoxicity. The exposure to respiratory sensitizers induced dendritic-like cell activation and a specific cytokine release pattern, while exposure to irritants did not. In addition, the cell surface marker OX40L was found to identify dendritic-like cell activation by high molecular weight allergens. With this in vitro model we can postulate a set of promising markers that allow the discrimination of chemical respiratory sensitizers from irritants.
Read the paper here.
PAG1 limits allergen-induced type 2 inflammation in the murine lung
Published July 18, 2019 Research group of Simon Phipps Institution: Imperial College London
Background Phosphoprotein associated with glycosphingolipid‐enriched microdomains 1 (PAG1) is a transmembrane adaptor protein that affects immune receptor signaling in T and B cells. Evidence from genome‐wide association studies of asthma suggests that genetic variants that regulate the expression of PAG1 are associated with asthma risk. However, it is not known whether PAG1 expression is causally related to asthma pathophysiology. Here, we investigated the role of PAG1 in a preclinical mouse model of house dust mite (HDM)‐induced allergic sensitization and allergic airway inflammation.
Methods Pag1‐deficient (Pag1−/−) and wild‐type (WT) mice were sensitized or sensitized/challenged to HDM, and hallmark features of allergic inflammation were assessed. The contribution of T cells was assessed through depletion (anti‐CD4 antibody) and adoptive transfer studies.
Results Type 2 inflammation (eosinophilia, eotaxin‐2 expression, IL‐4/IL‐5/IL‐13 production, mucus production) in the airways and lungs was significantly increased in HDM sensitized/challenged Pag1−/− mice compared to WT mice. The predisposition to allergic sensitization was associated with increased airway epithelial high‐mobility group box 1 (HMGB1) translocation and release, increased type 2 innate lymphoid cells (ILC2s) and monocyte‐derived dendritic cell numbers in the mediastinal lymph nodes, and increased T‐helper type 2 (TH2)‐cell differentiation. CD4+ T‐cell depletion studies or the adoptive transfer of WT OVA‐specific CD4+ T cells to WT or Pag1−/− recipients demonstrated that the heightened propensity for TH2‐cell differentiation was both T cell intrinsic and extrinsic.
Conclusion PAG1 deficiency increased airway epithelial activation, ILC2 expansion, and TH2 differentiation. As a consequence, PAG1 deficiency predisposed toward allergic sensitization and increased the severity of experimental asthma.
Key words HMGB1; PAG1; TH2 cells; house dust mite; type 2 inflammation
Read the paper here.
Lung responses in murine models of experimental asthma: value of house dust mite over ovalbumin sensitization

Published in Respir Physiol Neurobiol. 2018 Jan;247:43-51. doi: 10.1016/j.resp.2017.09.001. Epub 2017 Sep 8.
Abstract Ovalbumin (OVA) sensitization has limitations in modelling asthma. Thus, we examined the value of allergic sensitization using a purified natural allergen, house dust mite (HDM), over the sensitization performed with OVA. Mice were sham-treated, or sensitized with OVA- or HDM with identical chronology. Airway resistance, tissue damping and elastance were assessed under control conditions and after challenging the animals with methacholine (MCh) and the specific allergen. Inflammatory profile of the bronchoalveolar lavage fluid was characterized and lung histology was performed. While no difference in the lung responsiveness to the specific allergen was noted, hyperresponsiveness to MCh was observed only in the HDM-sensitized animals in the lung peripheral parameters. Lung inflammation differed between the models, but excessive bronchial smooth muscle remodelling occurred only with OVA. In conclusion, we demonstrate that a purified natural allergen offers a more relevant murine model of human allergic asthma by expressing the key features of this chronic inflammatory disease both in the lung function and structure.
Keywords Airway hyperresponsiveness; Allergen exposure; Animal models; Asthma; Mice.
Read the paper here.
Ezh2 controls development of natural killer T cells, which cause spontaneous asthma-like pathology

Background Natural killer T (NKT) cells express a T-cell receptor that recognizes endogenous and environmental glycolipid antigens. Several subsets of NKT cells have been identified, including IFN-γ-producing NKT1 cells, IL-4-producing NKT2 cells, and IL-17-producing NKT17 cells. However, little is known about the factors that regulate their differentiation and respective functions within the immune system.
Objective We sought to determine whether the polycomb repressive complex 2 protein enhancer of zeste homolog 2 (Ezh2) restrains pathogenicity of NKT cells in the context of asthma-like lung disease.
Methods Numbers of invariant natural killer T (iNKT) 1, iNKT2, and iNKT17 cells and tissue distribution, cytokine production, lymphoid tissue localization, and transcriptional profiles of iNKT cells from wild-type and Ezh2 knockout (KO) iNKT mice were determined. The contribution of NKT cells to development of spontaneous and house dust mite-induced airways pathology, including airways hyperreactivity (AHR) to methacholine, was also assessed in wild-type, Ezh2 KO, and Ezh2 KO mice lacking NKT cells.
Results Ezh2 restrains development of pathogenic NKT cells, which induce spontaneous asthma-like disease in mice. Deletion of Ezh2 increased production of IL-4 and IL-13 and induced spontaneous AHR, lung inflammation, mucus production, and IgE. Increased IL-4 and IL-13 levels, AHR, lung inflammation, and IgE levels were all dependent on iNKT cells. In house dust mite-exposed animals Ezh2 KO resulted in enhanced AHR that was also dependent on iNKT cells.
Conclusion Ezh2 is a central regulator of iNKT pathogenicity and suppresses the ability of iNKT cells to induce asthma-like pathology.
Read the full paper here.
Targeting the ICOS/ICOS-L pathway in a mouse model of established allergic asthma disrupts T follicular helper cell responses and ameliorates disease
Background Allergic asthma is characterized by chronic inflammation and remodelling of the airways, associated with dysregulated type 2 immune responses and allergen-specific IgE. T follicular helper cells (TFH ) are crucial in T-dependent B-cell responses and have been implicated in allergic airway disease (AAD). TFH , unlike other CD4+ T cells, are uniquely reliant on continuous ICOS signalling to maintain their phenotype after T-cell priming; therefore, disrupting this signal can impair TFH responses. However, the contribution of TFH to disease during chronic aero-allergen exposure and the therapeutic potential of targeting these cells have not been evaluated.
Methods To establish AAD, female BALB/c mice were repeatedly exposed to house dust mite or Alternaria alternata three times a week for up to 5 weeks. To examine the impact of TFH on AAD, mice were allergen exposed for 5 weeks and co-administered anti-ICOS Ligand-targeted antibodies, three times a week for the last 2 weeks.
ResultsTFH were first observed in the lung-draining lymph nodes and with further exposure were also found locally within the lungs. TFH accumulated with sustained allergen exposure, alongside germinal centre (GC) B cells. Blockade of ICOS signalling after AAD establishment successfully depleted TFH but did not affect the differentiation of other CD4+ T-cell subsets. This reduced GC responses, allergen-specific IgE, inflammation, pulmonary IL-13 and airway hyper-responsiveness.
Conclusions TFH are crucial in the regulation of AAD and the ICOS/ICOS-L pathway could represent a novel therapeutic target in allergic asthma.
Read the full paper here.
GSTO1‐1 is an upstream suppressor of M2 macrophage skewing and HIF‐1α‐induced eosinophilic airway inflammation
Background Glutathione S‐transferases omega class 1 (GSTO1‐1) is a unique member of the GST family regulating cellular redox metabolism and innate immunity through the promotion of LPS/TLR4/NLRP3 signalling in macrophages. House dust mite (HDM) triggers asthma by promoting type 2 responses and allergic inflammation via the TLR4 pathway. Although linked to asthma, the role of GSTO1‐1 in facilitating type 2 responses and/or HDM‐driven allergic inflammation is unknown.
Objective To determine the role of GSTO1‐1 in regulating HDM‐induced allergic inflammation in a preclinical model of asthma.
Methods Wild‐type and GSTO1‐1‐deficient mice were sensitized and aeroallergen challenged with HDM to induce allergic inflammation and subsequently hallmark pathophysiological features characterized.
Results By contrast to HDM‐challenged WT mice, exposed GSTO1‐1‐deficient mice had increased numbers of eosinophils and macrophages and elevated levels of eotaxin‐1 and ‐2 in their lungs. M1 macrophage‐associated factors, such as IL‐1β and IL‐6, were decreased in GSTO1‐1‐deficient mice. Conversely, M2 macrophage factors such as Arg‐1 and Ym1 were up‐regulated. HIF‐1α expression was found to be higher in the absence of GSTO1‐1 and correlated with the up‐regulation of M2 macrophage markers. Furthermore, HIF‐1α was shown to bind and activate the eotaxin‐2 promotor. Hypoxic conditions induced significant increases in the levels of eotaxin‐1 and ‐2 in GSTO1‐deficient BMDMs, providing a potential link between inflammation‐induced hypoxia and the regulation of M2 responses in the lung. Collectively, our results suggest that GSTO1‐1 deficiency promotes M2‐type responses and increased levels of nuclear HIF‐1α, which regulates eotaxin (s)‐induced eosinophilia and increased disease severity.
Conclusion & Clinical Implication We propose that GSTO1‐1 is a novel negative regulator of TLR4‐regulated M2 responses acting as an anti‐inflammatory pathway. The discovery of a novel HIF‐1α‐induced eotaxin pathway identifies an unknown connection between hypoxia and the regulation of the severity of allergic inflammation in asthma.
Read the full paper here.
