Reconstituting your allergens
Reconstituting your allergens
Our allergens are freeze-dried and produced without any preservatives or buffer salts. This allows you to reconstitute them in a buffer of your choice. We recommend a phosphate buffer with sodium chloride (phosphate buffered saline, PBS) as it offers the stability of the buffering capacities of the phosphate ion. It has many uses because it is isotonic and non-toxic to most cells. The uses include substance dilution and cell container rinsing. The optimal pH for cells is about pH 7.4 and this can be perfectly achieved with PBS.
However many scientist prefer to make aliquots of different samples and want to freeze them. In this case we recommend a simple saline water solution (0.9% NaCl) as PBS can form concentrated phosphoric acid during freezing. This can alter protein functionality of the allergen after freezing and thawing due to the forming of concentrated phosphoric acid.
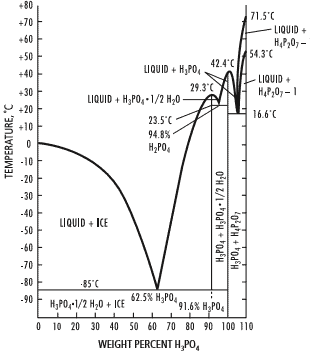
The phase diagram in Figure 1 of phosphate and water shows that during freezing, pure water is crystallised to pure ice. At the same time, phosphate is binding water causing a supercool liquid to become available in which the phosphate is concentrated to a phosphoric acid with water. At -85°C there is a liquid which consist of water and 62,5 % phosphoric acid which could affect the proteins in the frozen liquids.
So our advice is to avoid risks caused by the formation of phosphoric acid and therefor never use a phosphate buffer when freezing reconstituted allergens. Hence as an alternative, we suggest to use a simple saline water solution (0.9% NaCl) instead.
Sources
-1- Protein denaturation during freezing and thawing in phosphate buffer systems: monomeric and tetrameric beta-galactosidase. By Pikal-Cleland KA1, Rodríguez-Hornedo N, Amidon GL, Carpenter JF.
https://www.ncbi.nlm.nih.gov/pubmed/11368330
-2- Impact of freezing on pH of buffered solutions and consequences for monoclonal antibody aggregation. Kolhe P1, Amend E, Singh SK
https://www.ncbi.nlm.nih.gov/pubmed/20039442
-3- Crystallization-relatedpH changes during freezing of sodium phosphate buffer solutions.
Gomez, Gerardo
https://deepblue.lib.umich.edu/handle/2027.42/129526
-4- Quality-by-Design for Freeze-Thaw of Biologics: Concepts and Application to Bottles of Drug Substance By Angela Kantor, Serguei Tchessalov, Ph.D. Nicholas Warne, Ph.D.
https://www.americanpharmaceuticalreview.com/Featured-Articles/36901-Quality-by-Design-for-Freeze-Thaw-of-Biologics-Concepts-and-Application-to-Bottles-of-Drug-Substance/
