Buffers containing phosphoric ions
In short: Buffers using phosphates, such as the commonly used phosphate buffered saline (PBS), are frequently used in biochemical research. When your intentions is to freeze a product for long-term storage, we advise against using a phosphate buffer but suggest using a saline (0.9% NaCl) instead. During freezing, phosphoric acid is formed and could negatively affect your protein stability.
A commonly used isotonic buffer is based on phosphate, such as a phosphate buffered saline solution (PBS). The advantages of this buffer are its stability, buffering capacity and non-toxicity to most cells. Applications vary from washing cells, reconstitution of reagents and dilutions. The pH of 7.4 that is required for cells is easily achieved with phosphate buffers.
Freeze-thawing effects
The biggest disadvantage of phosphate buffers is one that is unknown to many researchers. During freezing, phosphoric acid can be formed which can negatively affect your reagents (be it proteins or chemicals). This can cause the reagents to loose functionality after freeze-thaw cycles.
What happens during freeze-thawing are known physics and can be best visualised in a phase diagram of an aqueous phosphate solution:
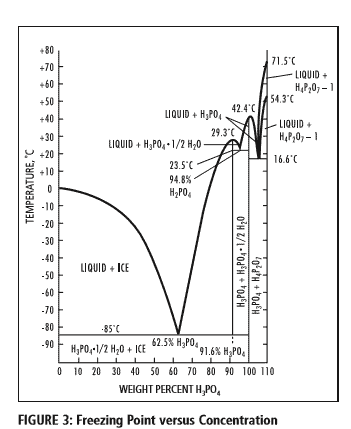
Figure 3: The phase diagram of the H3PO4 -H2O system showing the freezing point curve for phosphoric acid through the range of 0 to 100 weight percent H3PO4. Concentrated phosphoric acid tends to supercool before crystallization occurs. The freezing points of standard concentrations are as follows: Acid Strength(% H3PO4) 75% 80% 85% with freezing points 17.5°C 4.6°C 21.1°C respectively. Source: PotashCorp, IL, US.
What if you use a PBS buffer
If you are going to use PBS buffer, you can store it in a refrigerator at 2-8ºC. The time you can store it depends on the quality of your PBS and how sterile you are working. If you really want to freeze the solution we recommend to use 0.9% NaCl. In general, we recommend to freeze-thaw your material as little as possible.
Literature
This phenomenon is described in different articles see
- https://www.ncbi.nlm.nih.gov/pubmed/11368330 Protein denaturation during freezing and thawing in phosphate buffer systems: monomeric and tetrameric beta-galactosidase. By Pikal-Cleland KA1, Rodríguez-Hornedo N, Amidon GL, Carpenter JF.
- https://www.ncbi.nlm.nih.gov/pubmed/20039442 Impact of freezing on pH of buffered solutions and consequences for monoclonal antibody aggregation. Kolhe P1, Amend E, Singh SK
- https://deepblue.lib.umich.edu/handle/2027.42/129526 Crystallization-relatedpH changes during freezing of sodium phosphate buffer solutions. Gomez, Gerardo
- https://www.americanpharmaceuticalreview.com/Featured-Articles/36901-Quality-by-Design-for-Freeze-Thaw-of-Biologics-Concepts-and-Application-to-Bottles-of-Drug-Substance/ Quality-by-Design for Freeze-Thaw of Biologics: Concepts and Application to Bottles of Drug Substance By Angela Kantor, Serguei Tchessalov, Ph.D. Nicholas Warne, Ph.D.
